How We Helped a Leading US Clinical Research Company Improve Compliance Training with a Custom LMS
Overview
A major clinical research organization in the United States faced a growing challenge in managing oboarding and compliance training for over 1,000 geographically distributed employees. With strict regulatory requirements and constant updates to policies, the company needed a centralized, scalable, and easy-to-manage Learning Management System (LMS).
We implemented a fully customized LMS solution built on MoodleTM, tailored to their compliance needs and integrated with their existing enterprise systems.
Project Name
LMS for Clinical Research Company
Services
Setup, Development, Support
Type
LMS Development
The Challenge
Before implementing the new LMS, the client faced multiple challenges that hindered efficiency and compliance:
Manual Onboarding – New employees had to be trained manually by HR, leading to delays and inconsistencies.
Scattered Compliance Tracking – Compliance courses needed to be reset every year, but manual resets were error-prone and lacked proper history tracking.
Lack of Automation – Employees did not automatically receive courses based on their roles or departments. HR intervention was required each time an employee moved to a new position.
Limited Integration – Existing HR and IT systems such as Microsoft AD and Paychex were not connected to training data, creating silos of information.
Poor Visibility for Executives – Leadership teams did not have real-time insights into compliance status, training completions, or risk areas.
Geographically Distributed Workforce – With staff spread across the United States, consistent training delivery was difficult.
In short, the company needed a solution that combined automation, compliance assurance, and integration with its existing enterprise systems.

Our Solution
We implemented a MoodleTM-based LMS tailored specifically to the needs of clinical research compliance. The deployment focused on four pillars: automation, integration, compliance, and reporting.
1. Custom Theme & Branding
A clean, modern design was developed on top of MoodleTM to reflect the company’s brand identity. The LMS was fully aligned with corporate colors, typography, and branding guidelines, ensuring employees felt the platform was a natural extension of the organization rather than an external system.
2. Automated Onboarding Workflow
We created a structured onboarding program using MoodleTM courses. When employees joined, they were automatically enrolled in an onboarding track where they:
– Reviewed company policies
– Completed required compliance modules
– Uploaded required HR documents
– Signed acknowledgment forms
This workflow eliminated redundant HR tasks and ensured every new hire was compliance-ready from Day 1.
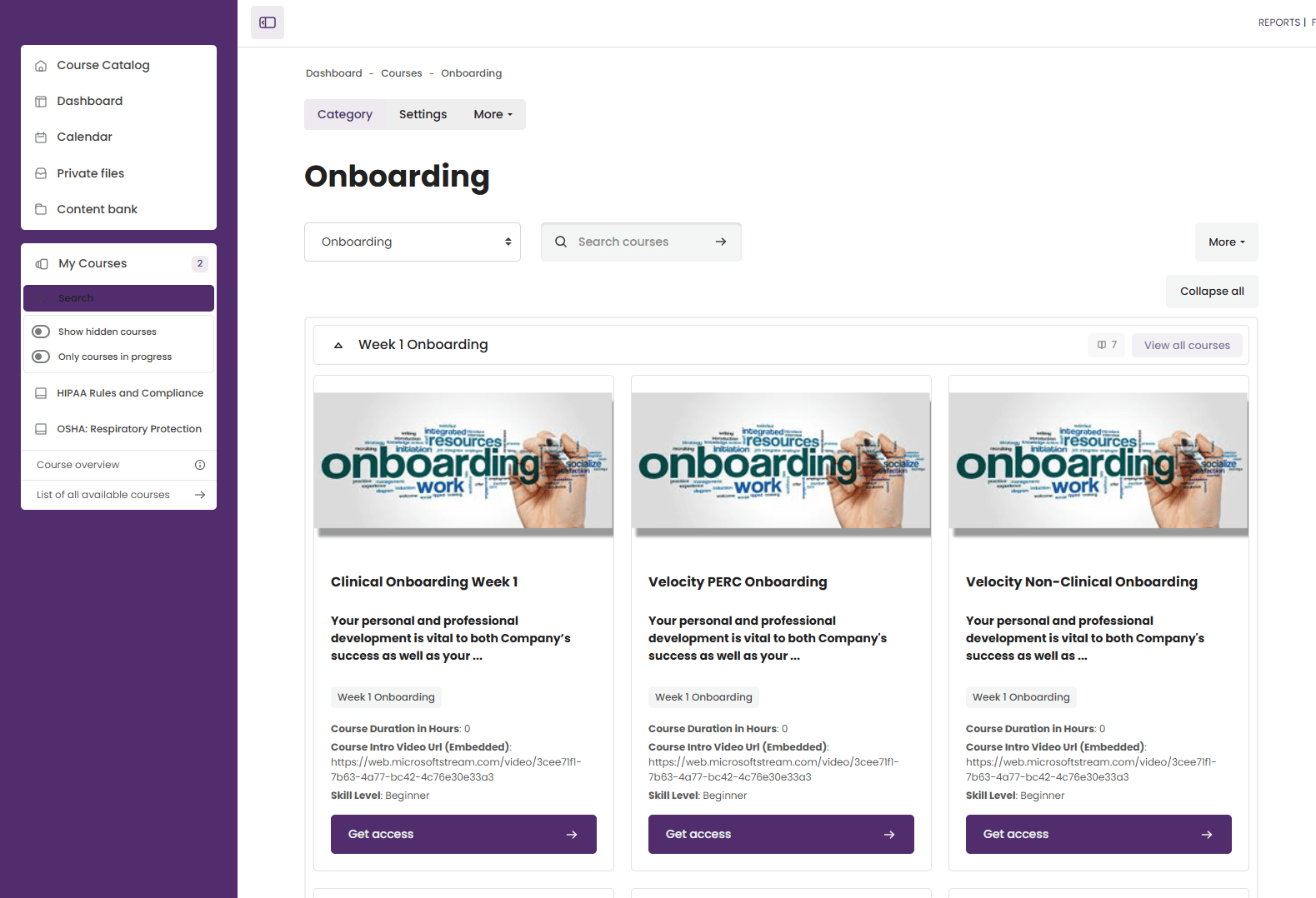
3. Roles, Cohorts, and Auto-Assignment of Courses
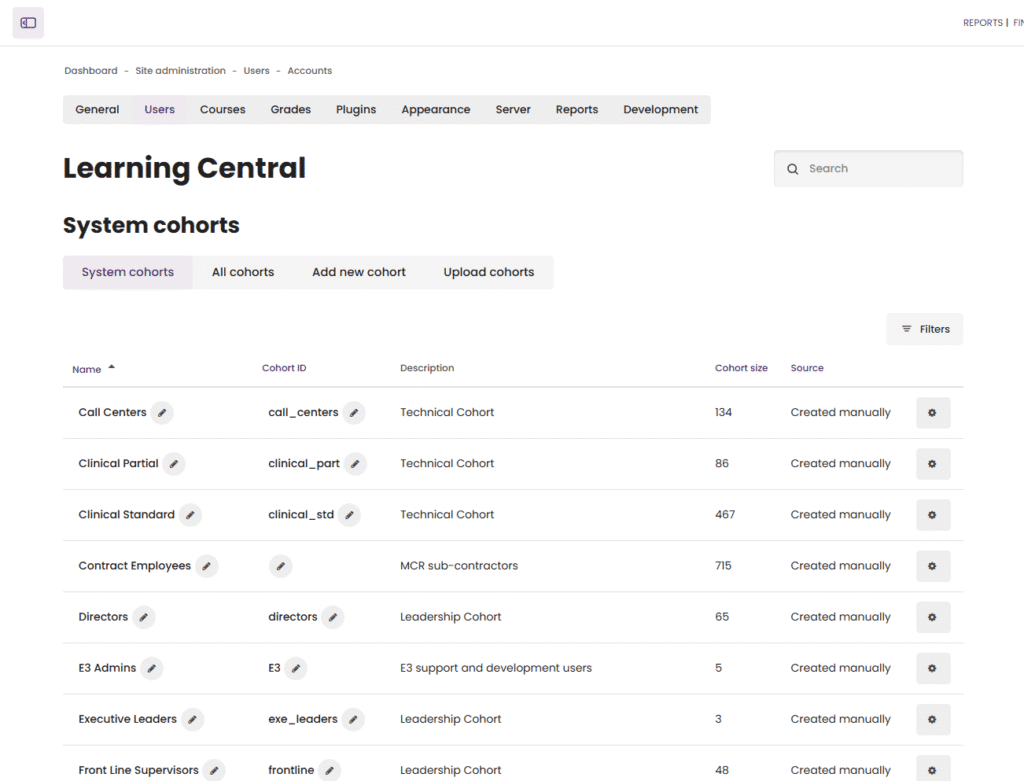
One of the most impactful features we introduced was the role and cohort-based auto-assignment system. Employees were grouped into cohorts based on department, role, or job function. The system was configured to:
Automatically assign mandatory courses based on an employee’s role
Instantly update course assignments if an employee moved departments or changed roles
Remove the need for manual HR intervention in assigning training
This automation ensured that the right people received the right training at the right time, reducing compliance gaps and saving HR significant administrative time.
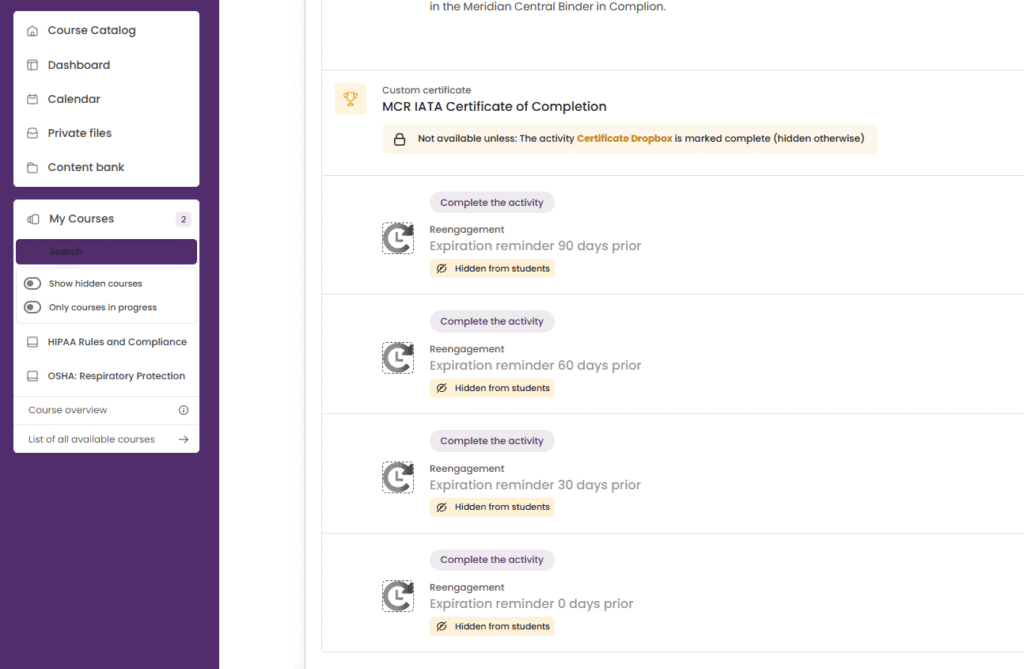
4. Compliance Training & Yearly Resets
Compliance courses were set up with annual reset cycles. At the end of each compliance year:
Courses were reset while preserving historical data for audits
Employees were automatically re-enrolled
Automated reminders were sent at 90, 60, 30, and 1 days before expiry
This feature significantly improved compliance rates by keeping employees consistently engaged and aware of deadlines.
5. Rich Training Formats
The LMS supported multiple training content formats, including:
SCORM packages for compliance modules
H5P interactive modules for active learning
Video lessons for policies and soft skills
PDF and document uploads for policy acknowledgment
This variety ensured engaging learning experiences across multiple learning styles.
6. Seamless System Integrations
We integrated the LMS with key enterprise systems:
Microsoft Active Directory (AD): Enabled Single Sign-On (SSO) for one-click access, eliminating the need for separate credentials.
Paychex HRM: Used webhooks to sync employee information on demand. This avoided heavy server loads compared to periodic API calls.
Microsoft SharePoint: Compliance and completion reports were auto-generated and uploaded to SharePoint folders for executive access.
Power BI: Data was connected to Power BI dashboards for advanced data visualization and predictive analytics.

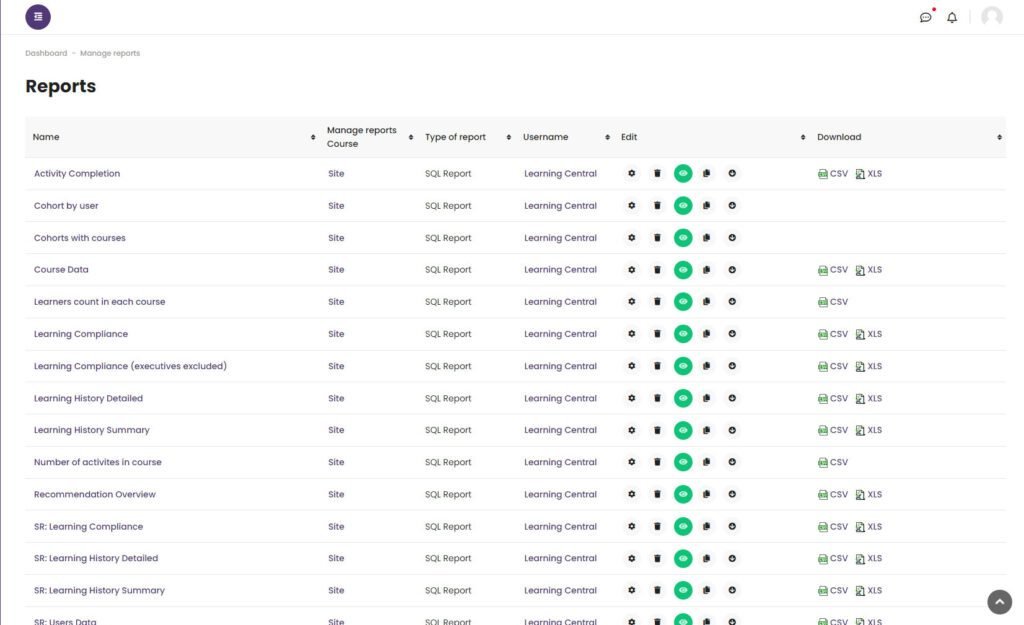
7. Advanced Reporting & Analytics
We provided a wide set of ad-hoc reports, including:
– Compliance tracking reports
– Course completion status
– User activity patterns
– Department-wise training performance
These reports were critical for audit readiness and helped executives quickly identify areas of risk.
8. Training & Documentation
To ensure smooth adoption, we created comprehensive user manuals and delivered training sessions to the Learning & Development department. This enabled the internal team to manage the LMS independently without relying on external vendors for day-to-day operations.
Team
1 Frontend developer, 1 Backend developer, 1 Quality Analyst, Project Manager.
Technologies
PHP, MySQL, MoodleTM, JavaScript, Apache HTTP Server, AWS, Amazon EC2, RDS, AWS Elastic Beanstalk , Microsoft Graph API, Paychex HRM Webhooks API
Have a project that you would like discuss?
Results & Impact
The results of the LMS implementation were transformative:
95%+ Compliance Completion Rates
Employees consistently completed their mandatory compliance courses, reducing regulatory risks.HR Efficiency Improved
Automated onboarding, cohort-based assignments, and yearly compliance resets significantly reduced manual HR workload.Employee Engagement Boosted
Timely reminders and engaging learning formats increased participation and completion rates.Executive Visibility Enhanced
With reports accessible directly in SharePoint and Power BI, leadership could monitor compliance in real-time.Scalable Infrastructure
The LMS was designed to scale with the organization, ensuring that additional employees, departments, or compliance requirements could be handled seamlessly.Audit-Ready at All Times
With historical data preserved, the company was always ready for external audits without last-minute scrambles.
Why It Matters for Clinical Research
Clinical research companies operate in one of the most compliance-heavy industries in the world.
Mistakes in training management can lead to:
Failed regulatory inspections
Delayed clinical trials
Financial and reputational losses
By implementing a customized LMS for compliance training, the company not only ensured regulatory readiness but also positioned itself for sustainable growth.
The solution became a single source of truth for training and compliance data, bridging HR, IT, and executive leadership needs.
Conclusion
Through a carefully designed and implemented MoodleTM-based LMS, we helped one of the largest clinical research organizations in the US transform its compliance and training process. By combining automation, integrations, and reporting, the LMS streamlined onboarding, ensured 95%+ compliance rates, and gave executives unparalleled visibility into workforce training.
This project demonstrates how a well-implemented LMS can go beyond training delivery — becoming a powerful tool for compliance management, employee engagement, and organizational efficiency in regulated industries like clinical research.








